Real-world progression-free and overall survival of patients with metastatic colorectal cancer according to first- and second-line treatment regimen: PROMETCO study
Data presented at the ESMO-GI Congress 2024
Miriam Koopman,1 Rocio Garcia-Carbonero,2 Carmine Pinto,3 Andrey Mitroshkin,4 György Bodoky,5 Francisca Marti Marti,6 Juan Manuel O’Connor,7 Stanislav John,8 Joseph Sgouros,9 Helder Mansinho,10 Richard Greil,11 Pieter-Jan Cuyle,12 Stjepko Plestina,13 Janja Ocvirk,14 Adam Sullivan,15 Elias Choucair,16 Bénédicte Chevallier,16 Jean-Baptiste Bachet17
1. Department of Medical Oncology, University Medical Centre Utrecht, Utrecht University Heidelberglaan 100 3584 CX Utrecht, the Netherlands; 2. Oncology Department, Hospital Universitario Doce de Octubre, Imas12, Departamento de Medicina, Universidad Complutense de Madrid (UCM), Avenida De Córdoba s/n, 28041 Madrid, Spain; 3. Medical Oncology, Comprehensive Cancer Centre, AUSL –IRCCS di Reggio Emilia –Viale Risorgimento, 80 42123 Reggio Emilia, Italy; 4. Klinikum Freudenstadt, Akademisches Lehrkrankenhaus der Universität Tübingen, Karl-von-Hahn-Strasse, 100, 72250 Freudenstadt, Germany; 5. Dél-Pesti Centrumkórház Szent László Telephely Albert Flórián út 5–7 1097 Budapest, Hungary; 6. Department of Medical Oncology, The Christie NHS Foundation Trust, Wilmslow Road, Manchester, M20 4BX, UK; 7. Oncólogo Clínico MN102.684, Jefe Área Tumores Gastrointestinales, Instituto Alexander Fleming, Buenos Aires, Argentina; 8. Clinic of Oncology and Radiotherapy, University Hospital Hradec Králové, Sokolská 581, 500 05, Hradec Králové, the Czech Republic; 9. Department of Medical Oncology, Agii Anargiri General Hospital and Cancer Center, Athens, Greece; 10. Oncology Department, Garcia de Orta Hospital-Almada, Portugal; 11. Paracelsus Medical University Salzburg, Salzburg Cancer Research Institute-Center for Clinical Cancer and Immunology Trials, Cancer Cluster Salzburg, Austria; 12. Gastroenterology and Digestive Oncology Department, Imelda General Hospital, Bonheiden, Belgium; 13. Department of Oncology, University Hospital Centre Zagreb, Croatia; 14. Institute of Oncology Ljubljana, Zaloska 2, 1000 Ljubljana, University of Primorska, Faculty of Health Sciences, Polje 42, Slovenia; 15. Servier Pharmaceuticals, 200 Pier 4 Blvd, Boston, MA 02210, USA; 16. Servier, 35 rue Verdun, 92284 Suresnes, France; 17. Sorbonne Université, Service d’hépato-Gastro-Entérologie, Groupe Hospitalier Pitié Salpêtrière, APHP, Paris, France.
PROMETCO: Study background and aim
-
The clinical emphasis for treating unresectable mCRC focuses on avoiding rapid disease progression and prolonging survival1
-
Recent advances have improved mOS for patients with mCRC to 30 months in clinical trials,1 and data on later-line treatments like trifluridine/tipiracil + bevacizumab and fruquintinib suggest that this could be extended further2,3
-
The PROMETCO (NCT03935763) study is the first international, prospective, real-world study investigating the continuum of care in mCRC patients after two disease progressions since diagnosis
Aim: To assess real-world OS and PFS by first-line and second-line (1L and 2L) treatment regimen for the 655 of 738 patients with mCRC who completed the PROMETCO study
-
Inclusion criteria: Adult patients with two disease progressions since the first diagnosis of metastatic disease, suitable to receive subsequent treatment
-
Exclusion criteria: Patients enrolled in other clinical trials, receiving treatment for other cancers, or those with reduced mental capacity
Baseline data at diagnosis were collected before PROMETCO inclusion, and the prognostic subgroups were as defined by Tabernero et al. (2020)4
The data were analyzed by six different 1L and 2L treatment groups.
These groups are presented throughout:
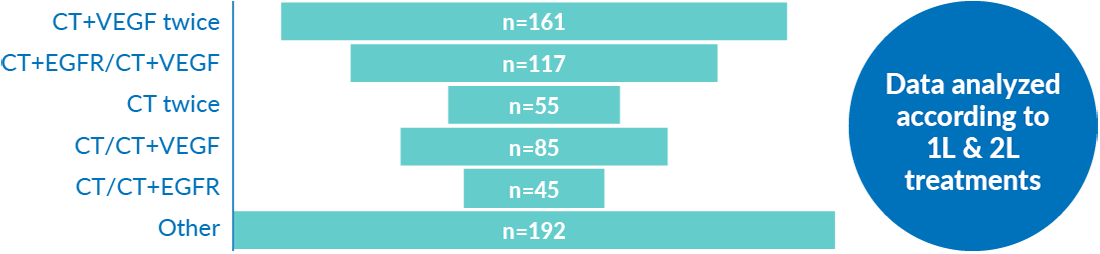
Patient characteristics (n=655)
Efficacy outcomes (n=655)
Conclusions
CT, doublet/triplet chemotherapy; EGFR, anti-epidermal growth factor receptor; mCRC, metastatic colorectal cancer; mOS, median OS; mPFS, median PFS; OS, overall survival; PFS, progression-free survival;
VEGF, anti-vascular endothelial growth factor.
1. Van Cutsem E, et al. Ann Oncol. 2016;27(8):1386–22. 2. Prager GW, et al. N Engl J Med. 2023;388(18):1657–67. 3. Dasari A, et al. Lancet. 2023;402(10395):41–53. 4. Tabernero J, et al. ESMO Open. 2020;5(4):e000752.
back to top

back to top


